
-
Dipl. Ing. (FH) Computer Science
- Dipl. Business Engineer NDS HF
- Certified Expert Quality Management Medical Devices International
SHORT SUMMARY OF PROFILE
- Dipl. Ing. (FH) in Informatics & Dipl. Business Engineer NDS HF (state diplomas recognized by Switzerland)
-
Expert Quality Management Medical Devices International
(TÜV Rheinland personnel certification no. 3094498 acc. to examination regulation ID 27230)
-
freelance specialist for Quality Management (QM) and compliant QM
processes in the medical device industry
(ISO 13485, 21 CFR Part 820, EU-MDR, et al.) since mid-2014
- CAPA SME (Subject Matter Expert)
-
4 years' experience as responsible Quality Management Representative in the medical device industry:
- achievement and preservation of ISO 9001 / ISO 13485 certification considering MDD (93/42/EEC) and GMP compliance to
gain CE certification for class IIa medical devices
- more than 10 years' experience as Coordinator and Project Manager for ERP system and organizational projects
FOCUS OF ACTIVITY AND CONSULTANCY
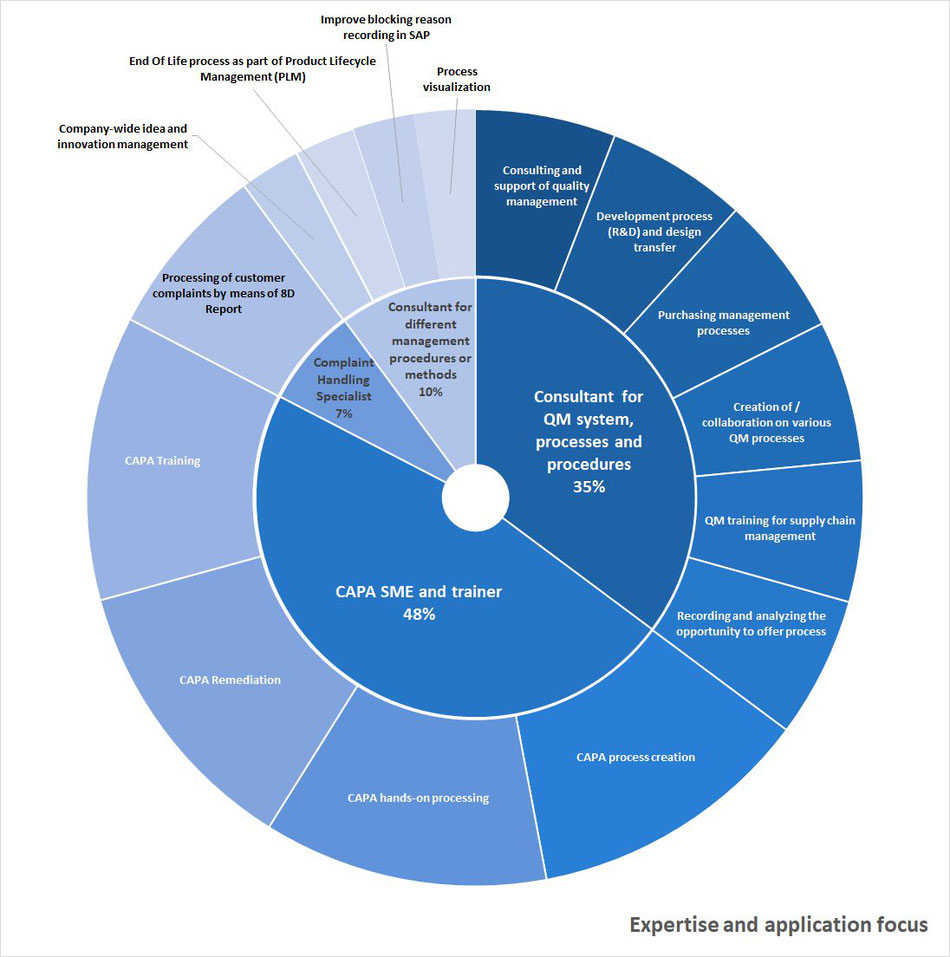
1. Establishment and maintenance of QM systems according to ISO 13485:2016 and 21 CFR 820 (QSR) in the medical device industry
- QM projects with a focus on processes and procedures:
-
-
Carrying out gap analyses of the QM system regarding compliance with regulatory requirements, such as ISO 13485, QSR or
EU regulation 2017/745 (EU MDR) - Implement regulatory compliant, efficient and effective processes, work instructions and templates individually adapted to company needs:
-
- Customer projects conducted for CAPA, R&D, design transfer and procurement processes
- Compilation of the technical documentation according to EU MDR Annex II and III for class I, Ir, Is, IIa and IIb products
-
Carrying out gap analyses of the QM system regarding compliance with regulatory requirements, such as ISO 13485, QSR or
2. CAPA SME (Subject Matter Expert)
- Hands on CAPA support, especially for extensive and complex CAPA
- CAPA remediation considering regulatory requirements
- CAPA process efficiency enhancement
- CAPA training for all experience levels
3. Customer complaint handling using 8D report
4. Project management and coordination for projects
- Focus on QM, organization and ERP projects
5. Holistic project management system
- Establish and implement
6. Business Process Management
- Process map creation (business, management and support processes)
- BPM 2.0 (Social Business Process Management)
7. Organizational development
INTER-PERSONAL SKILLS
- Troubleshooter
- Excellent analytical skills
- Analyze – Structure – Synthesize
- Entrepreneurial thinking and targeted action
- Strong social competences
- Strong team player and strong capability to work independently
- Quick wit, communicative, intuitive, visual thinking, proactive and imaginative
-
Constant search for new challenging tasks and to take on complex scopes
LANGUAGES
Language (level of experience)
- German (native language, including High German and Swiss German)
- English (fluent)
- French (intermediate)
- Italian (basic)
Strategic Focus.
Accurate Implementation.
Comprehensive Progress.
Success.
Impressum | Datenschutz | Cookie-Richtlinie | Sitemap
© COPYRIGHT 2014 - 2025: Alle Rechte vorbehalten / All rights reserved by Martin R. Goetschi Consulting
© COPYRIGHT 2014 - 2025: Alle Rechte vorbehalten / All rights reserved by Martin R. Goetschi Consulting
